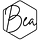Natural Bundle Dyeing - October Experiments with Iron
Using local Californian Native Plants
Mostly native plants. Bay Leaves - they’re definitely native. They grow everywhere. Everything else does grow here…but maybe not in the wild. In any case there I was mordanting some fabric (aka soaking it in stuff so the dye would “take” better) and I thought “I might as well throw in some white cotton I found laying about”. Some aluminium acetate later and I had a bag of bay leaves, carrot tops, avocado and pomegranate skin in the freezer, cochineal in a bag, and a spare hour. On the left you have the plant material, and on the right you have how it ended up.


Top right speckles are the cochineal bugs. Amazing things (“mexican red” - more about them here. Saw them living their little lives on cactus in Peru.) Top left green bits are the carrot tops. Little bit of pink in the midsection from the avocado (yes, it gives pink dye), and then the blue further down is either an unexpected effect from the rosemary or a little bit or iron impacting the cochineal. General greeny yellow is the bay leaves.
I took zero pics of the process but essentially I wet the fabric, rolled it up, and steamed it for 15 mins before allowing it to cool, unrolling and rinsing. (Of course you don’t use your usual pots and pans for this as natural things can be poisonous aka deadly nightshade, arsenic, all other members of the bay leaf family etc).
This first one was more by way of a “control” rather than an experiment since I was already familiar with the various elements and the colors they gave. What I was really interested in was stage 2. On the left we have the exact same plants but interspersed between them are bits of iron. Chains, nuts, bolts, springs, screws and so on.


Now this is what I’m talking about. I freakin ADORE the effect iron has on natural dye colors. This is what they should have been teaching in chemistry classes instead of covalent bonds and whatnot. Here are a few close ups.
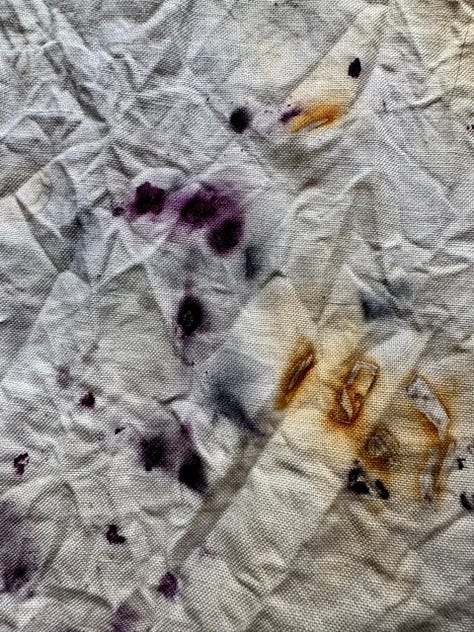

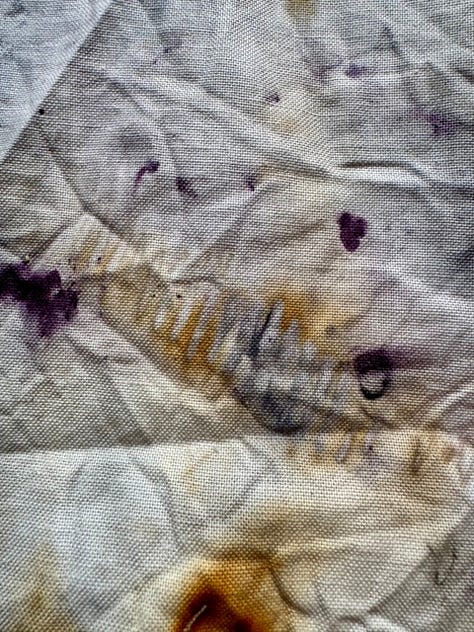



It’s the same cochineal causing the rich fuschia pink and the almost black purple. It’s just that the iron causes it to go so much darker. You can see the outlines of some of the iron pieces too (which is why I prefer using actual pieces to using ferrous sulphate generally). The brown is the rust from the iron. For this bundle I prepared it in the same way as the first one, but also dampened the fabric with white vinegar. After steaming for 15 mins I let it cool down enough to handle then put the whole lot still damp in a ziplock bag with an extra shot of vinegar for 2 days, during which time it got a lot darker and without which I wouldn’t have the rust areas. Rust needs time to develop and the acidic vinegar helps hugely.
I am in love with this piece of fabric.
I’ve been teaching natural bundle dyeing for a few years now but have definitely been doing more of it for use in my own work during the last few months. I can thoroughly recommend Rebecca Desnos as a resource and her guidance works worldwide, not just in her native England (which I wondered about as they have different plants in the UK, but an avocado is an avocado. Mostly.). And I do of course teach natural and unnatural dyeing myself - details here.
But, if you were paying close attention, you’ll be wondering about the actual fabric I meant t mordant, not this random white cotton I added. Well…that is for the first of the 6 Peru-inspired quilts I’m working on and I will show you the results of that next week.